-
Chemical recombination restricted by surrounding
hydrogen molecules
-
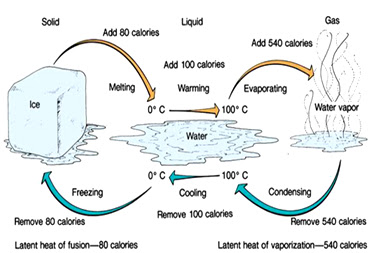
Ice density
0.9170 g/cm3 is ~8% lesser than liquid water
-
Salinity lowers freezing point, salt interferes
ordered crystal rearrangement
-
35‰ salinity with freezing point -1.91°C
-
Seawater contains dissolved substance
Salinity
-
Total solid material
dissolved, including dissolved gases, excluding suspended fine particles
Seawater constituents
-
70 elements, 6 major elements make 99%: Na, CI, SO4,
Mg, Ca, K
Salt Ion
|
Ions in Seawater
(‰)
|
Ions by Weight
(%)
|
Cumulative (%)
|
Chloride (Cl-)
|
18.980
|
55.04
|
55.04
|
Sodium (Na+)
|
10.556
|
30.61
|
85.65
|
Sulfate (SO42-)
|
2.649
|
7.68
|
93.33
|
Magnesium (Mg2+)
|
1.272
|
3.69
|
97.02
|
Calcium (Ca2+)
|
0.400
|
1.16
|
98.18
|
Potassium (K+)
|
0.380
|
1.10
|
99.28
|
Bicarbonate (HCO3-)
|
0.140
|
0.41
|
99.69
|
Bromide (Br-)
|
0.065
|
0.19
|
99.88
|
Boric Acid (H3BO3)
|
0.026
|
0.07
|
99.95
|
Strontium (Sr2+)
|
0.013
|
0.04
|
99.99
|
Fluoride (F-)
|
0.001
|
0.00
|
99.99
|
Total
|
34.482
|
99.99
|
99.99
|
Trace
Elements
- - 0.001‰; 1ppm
- - manganese (Mn), lead (Pb), gold (Au), iron (Fe), iodine (I)
Seawater
composition
- - Dissolved gases, Nutrient (N, Si, P), Organic compounds (fat, protein, carbohydrate)
- - NO3- and PO43- utilized by photosynthesizers
- - Biological uptake & release, non-conservative (highly varied [con])
Non-conservative
- - Seawater dissolved substances tied to biological/seasonal/geological cycles (short Residence Times)
Residence
Times
- - Avr. time a substance remains in specified region of space
Salinity
variation
- - Avr. 33 – 38ppt
- - Baltic Sea 10ppt
- - Red Sea & Persian Gulf 42ppt
- - Dead Sea 330ppt (hypersaline inland lake)
- - Great Salt Lake, Utah 280ppt (hypersaline inland lake)
Salinity
Determination
- - Evaporate weight amount of seawater & weigh salt
- - Ocean well-mixed; Exact same proportion of major dissolved constituents everywhere
- - 550.4‰ chlorinity; easy to measure
- - Salinity (‰) = 1.80655 x Chlorinity (‰) or conductivity
Conductivity
- - Measures strength of nutrient solution
Processes Decreasing
Salinity
-
Precipitation
-
Runoff
-
Melting
icebergs
-
Melting
sea ice
Salinity
regulator
- - River bring dissolved salts 2.5 – 4.5 x 1015 g/yr
- - Atmospheric volcanic gas & hydrothermal circulation release cations (Ca2+ , K+) and anions (SO42-, Cl-)
Chemical
equilibrium factors
- - Chemical precipitation & evaporite minerals (CaCO3, NaCl, CaSO4)
-
Evaporation & supersaturation
- - Marine aerosols
-
Salt coatings nearshore
- - Ion Adsorption by clay minerals (authigenic mineral formation)
-
Al, Fe, Mg
- - Biological precipitation
-
Hard
part secretion (Ca2+, Sr2+), Eg: shells
- - Drawn into mantle & subduction zone
Gas
|
Dry
Air (%)
|
Surface
Ocean (%)
|
Nitrogen
(N2)
|
78.03
|
47.5
|
Oxygen
(O2)
|
20.99
|
36.0
|
Carbon
Dioxide (CO2)
|
0.03
|
15.1
|
Hydrogen
(H2)
|
Trace
|
Trace
|
Argon
(Ar)
|
Trace
|
Trace
|
Neon
(Ne)
|
Trace
|
Trace
|
Helium
(He)
|
0.95
|
1.4
|
CO2
+ H2O + Light » CH2O + O2 (Photosynthesis)
CH2O + O2 » CO2 + H2O + Energy (Respiration)
CH2O + O2 » CO2 + H2O + Energy (Respiration)
-
Gas
diffusion across air-sea interface & planktonic photosynthesis (high 02)
-
Organic
matter accumulation, feeding organism respiration, bacterial decomposition (Low
02)
-
Low
BOD, cold gas-saturated water, hydrothermal vent (Increasing 02)
pH = - log10 [H+]
Seawater as
Buffer (pH 8)
-
CO2 + H2O à H2CO3
(Mostly)
-
H2CO3 à H+
+ HCO3- (Bicarbonate
– further dissociation)
-
HCO3- à H+
+ CO32- (Carbonate
– further dissociation)
-
CO32- +
Ca2+ --> CaCO3


No comments:
Post a Comment